The Vascular Care Group
Expertise
Experienced Regulatory Guidance Every Step of the Way
Vascular Breakthrough’s experts lead the way in navigating the guidelines for clinical trial conduct. As no two studies are the same, and the regulatory framework around trials is still evolving, Vascular Breakthroughs works with its partners on a regulatory strategy most appropriate for the therapeutic indication and the target market.
Clinical Trial Execution
With more than a decade of experience delivering clinical trials support, Vascular Breakthroughs brings together the end-to-end infrastructure and clinical expertise to deliver the full spectrum of research coordination and support, while providing flexibility to meet evolving patient and investigator needs.
Our pace of enrollment rivals, and often exceeds, that of many well-known academic centers. With an average of just 158 days from the start-up package to SIV, we're recognized as the top enroller in 83% of the trials we participate in, maintaining an exceptionally low 0.03% subject loss to follow-up.
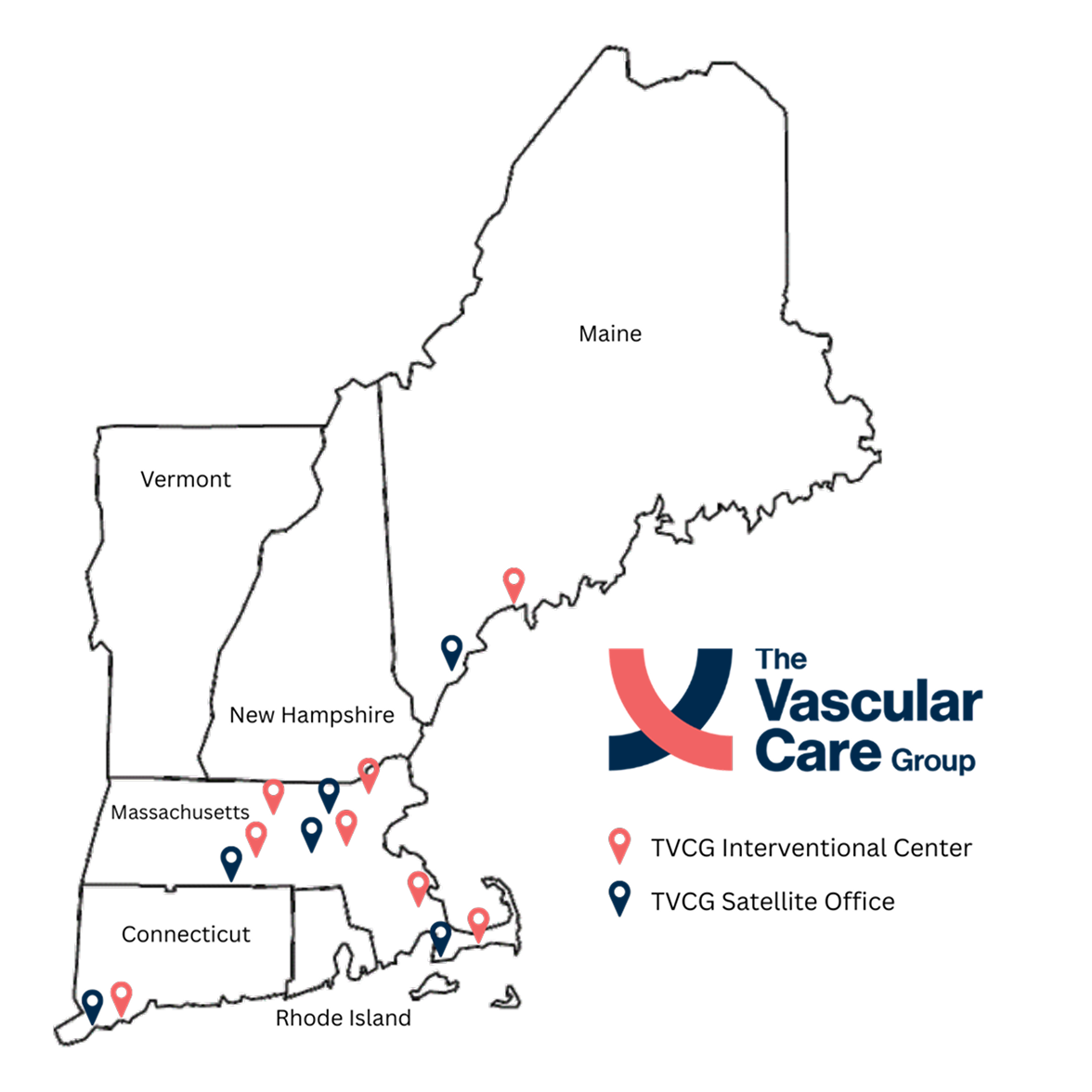
Leveraging our tools, experienced team, and expansive network of nearly 30 vascular specialists across New England, Vascular Breakthroughs delivers the speed, flexibility, and data integrity needed to drive next-generation vascular trials forward.
We provide:
Leveraging our tools, experienced team, and expansive network of nearly 30 vascular specialists across New England, Vascular Breakthroughs delivers the speed, flexibility, and data integrity needed to drive next-generation vascular trials forward.
We provide:
- Broad geographic area
- Diverse patient population and demographics
- Paperless platforms, 21 CFR 11 compliant enables clean, attributable data collection with ease of monitoring
- One budget/one contract can be applied to one or multiple study sites
CRO Support
At Vascular Breakthroughs, we have built outstanding relationships with many CROs in the industry. This collaboration offers sponsors a predictable pool of investigators and patient populations to meet recruitment goals in a timely manner.
Your Partner in Innovation
Vascular Breakthroughs has been screening, recruiting, and consenting patients for over 10 years. With this unique perspective, we can truly empathize with sites on the complexity required to turn a referral into a consented participant.
Vascular Breakthroughs:
- Facilitates collaboration and interactions between TVCG clinicians and our industry partners.
- Develops protocols for new indications for existing devices or new ideas altogether.
- Assists in funding support to get new projects off the ground.
- Assists in overcoming regulatory hurdles.
- Provides data analytics to plan protocols and trials for efficient enrollment to support new projects.

